Project 1 Organometallic Synthesis and Catalysis with Trianionic Pincer Ligands 
- Catalytic Cyclic Polymer Synthesis
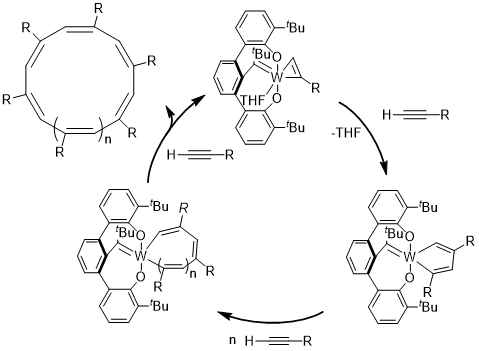
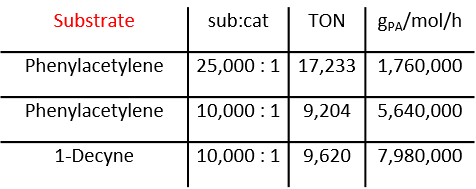
We discovered a highly active catalyst that is capable of polymerizing alkynes to give cyclic polyenes. Cyclic polymers exhibit remarkably different physical properties compared to their linear counterparts. Despite their interesting properties, laborious synthesis required for cyclic polymers have hindered development of this research field. Methods for the synthesis of cyclic polymers usually involve intramolecular coupling between the chain ends of linear precursors; however, the inherent limitation of this method is the requirement of dilute conditions and long reaction times. Recent discoveries in catalytic production of cyclic polymers offer exciting new avenues for their efficient production. Along with our polymer investigations we continue to develop more efficient , selective, and potentially living catalysts for cyclic polymer synthesis.
-
Highly Active M-C Multiple Bonds (Inorganic Enamine)
 Manipulating the electronic and geometric structure of transition metal complexes through judicious ligand design is central to the evolution of modern organometallic chemistry. Presented with a vast choice of ligand architectures, it is now simply a challenge of matching the correct ligand with a particular metal ion in the appropriate oxidation state to achieve the desired outcome. In particular, it is now possible to manipulate the nucleophilicity of metal-carbon multiple bonds by employing the inorganic enamine effect. Resonance structures reveal the relationship between enamines and an inorganic version. Applying this concept, alkylidyne complexes rapidly deoxygenate carbonyl complexes including CO2.
Manipulating the electronic and geometric structure of transition metal complexes through judicious ligand design is central to the evolution of modern organometallic chemistry. Presented with a vast choice of ligand architectures, it is now simply a challenge of matching the correct ligand with a particular metal ion in the appropriate oxidation state to achieve the desired outcome. In particular, it is now possible to manipulate the nucleophilicity of metal-carbon multiple bonds by employing the inorganic enamine effect. Resonance structures reveal the relationship between enamines and an inorganic version. Applying this concept, alkylidyne complexes rapidly deoxygenate carbonyl complexes including CO2. - Alkylidyne Complexes as REMP Initiators
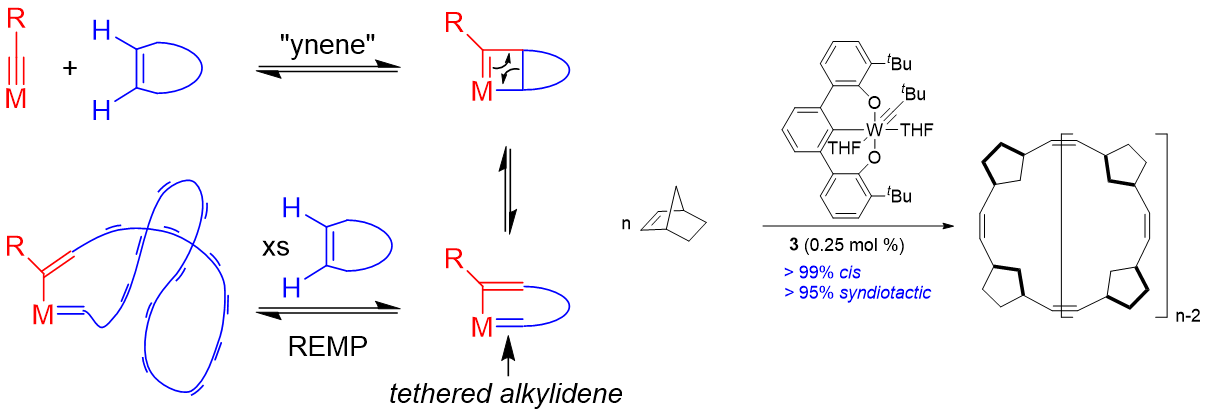
Enyne metathesis involves the reaction between an alkylidene and an alkyne. Numerous ruthenium carbenes and early metal alkylidenes react with alkynes. However, alkylidynes do not react with alkenes in “ynene” metathesis. Trianionic pincer ligands can induce unusually nucleophilic metal-carbon triple bonds. Thus, we hypothesized that if a trianionic pincer alkylidyne could be coaxed to undergo cycloaddition with a cyclic olefin it would inherently lead to a tethered alkylidene, and therefore poised to initiate ring expansion metathesis polymerization (REMP) to give cyclic polymers. The OCO trianionic W-complex depicted initiates the stereosective REMP of norbornene to give >99{815265df4c373d3cda1a2b97881ad12b4e5add1c8ca6f2d2b45936aa6e519af3} cis and >995 syndiotactic cyclic polynorbornene.
Project 2 Inorganic Click (iClick)
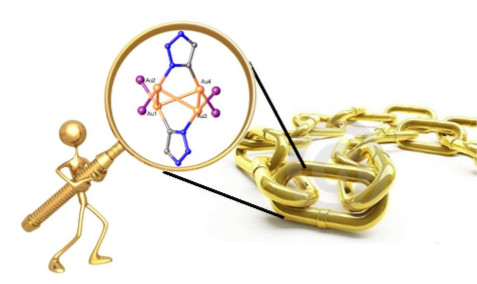
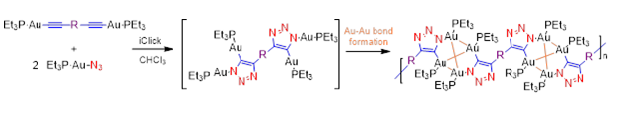
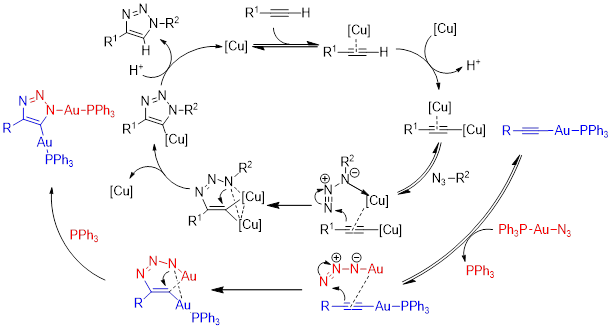
This project takes advantage of a recent discovery in the Veige research group involving the 1,3-dipolar cycloaddition of metal-azides and metal-acetylides to give dinuclear-1,2,3-triazolates. The first inorganic click (iClick) reaction was successfully accomplished in the Veige laboratories in 2010. iClick covalently links metal ions through a triazolate bridge in the 1,5 positions to give homo-/heterobimetallic units that can form oligomers/polymers. Additionally, iClick has been extended to include several transition metals: Au, Pt, Ir, and Rh. Conjugated polymers incorporating metal ions in the main chain are important for their ability to delocalize excitons to create charge separation in the polymer. Conjugated polymers see use in OLEDs, chemical/physical sensing, and photovoltaics. In this project we have synthesized gold oligomers using iClick and aurophilic cluster formation. The formation of the cluster is reversible so we have the ability to tune the metallopolymer to give different properties such as absorption/emission and sensing.
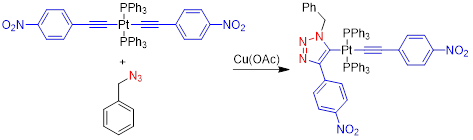
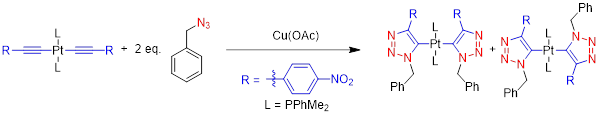
We have synthesized Pt molecules using iClick and can control the number of cycloadditions. We are building off these conclusions to synthesize Pt oligomers/polymers. Additionally, we have mechanistic data that indicates the iClick mechanism mirrors the copper-catalyzed azide-alkyne cycloaddition (CuAAC) reaction.
Project 3 Organometallic Chemistry Applied to Personalized Medicine (Aptamer-NHC)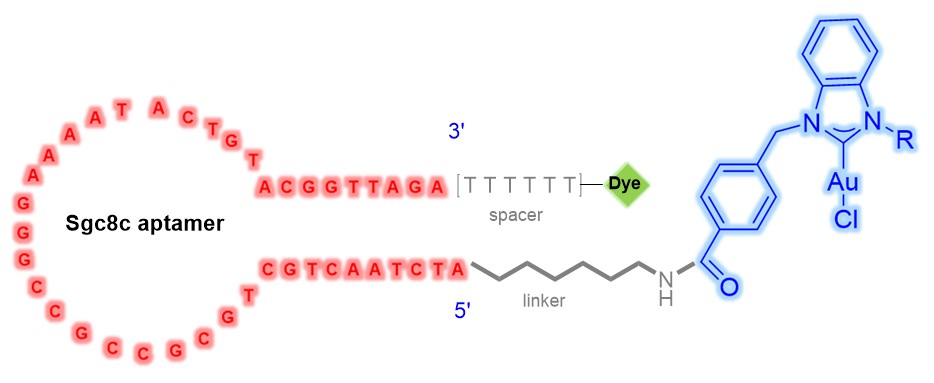
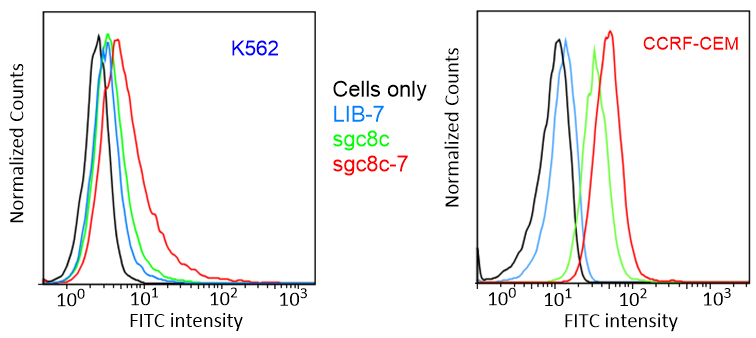
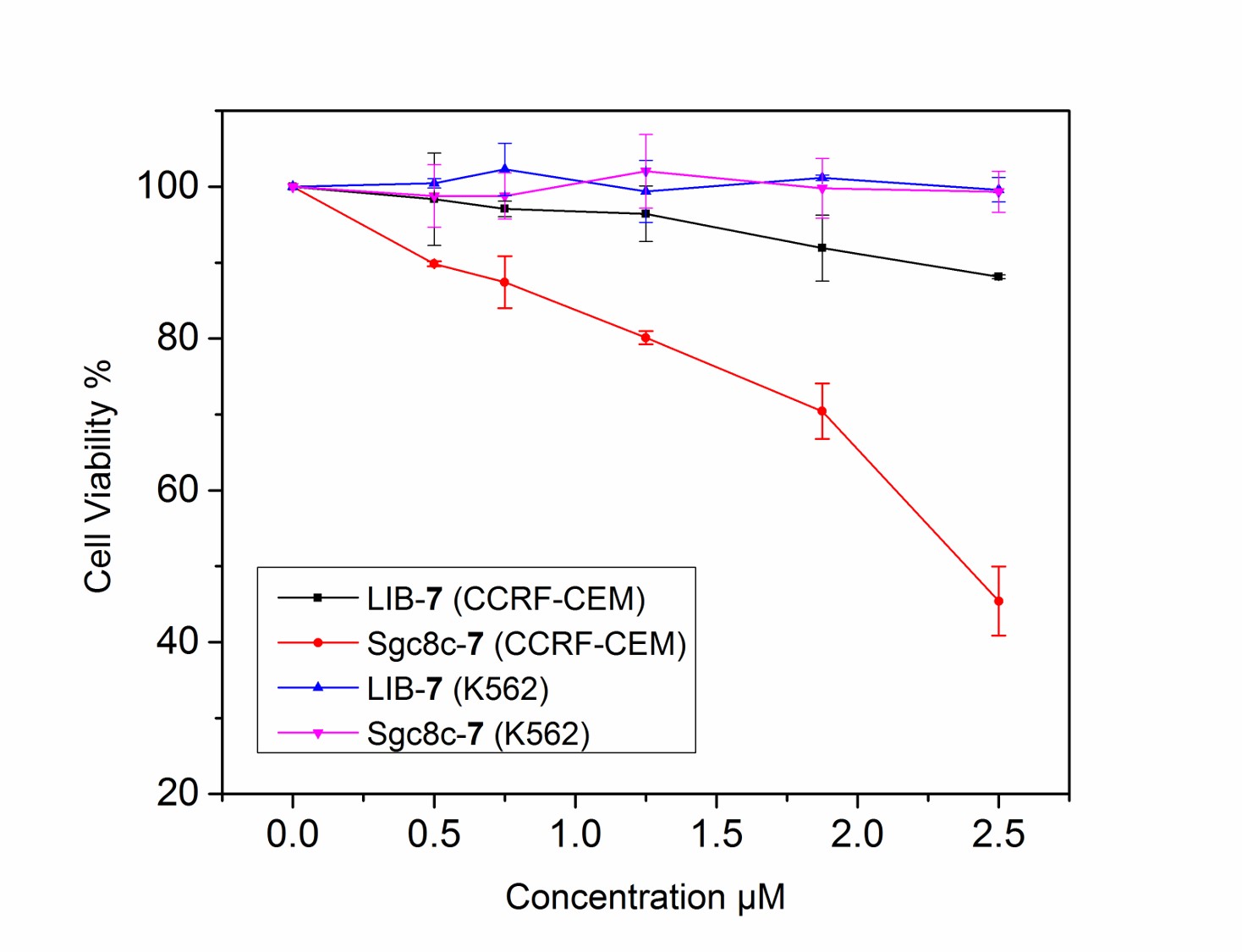
The idea for this project is that biomarkers (proteins expressed by the cancer cell) specific to the cancer and even to the individual’s particular cancer will be exploited to deliver chemotherapeutics. This proposal will exploit highly selective cancer-specific biomarkers to deliver NHC-Au complexes as the cytotoxic agent. Execution of the delivery vehicle is already complete; cell-specific aptamers with a high affinity for leukemia, liver, lymphoma, colon, and breast cancer are known. The ideal cytotoxic agent has yet to be discovered. We propose to conjugate metal complexes to cancer specific aptamers. Our approach will ultimately lead to patient-specific therapies. Individual patients will be able to have their specific cancer cell profiled and then a specific aptamer will be prepared that will recognize that patients cancer cell. The biomarkers are specific to each patient and the aptamers will be used to deliver a payload of a cytotoxic metal ion to the cancer cell to induce apoptosis. By using patient specific aptamers ultra low dosages of drug therapies will be required, therefore inherently reducing threatening toxic side-effects. Chemistry will be developed to synthesize new cytotoxic metal complexes and new bioconjugation strategies to attach the metal complex to the cell-specific aptamer. The ultimate goal is to develop one agent that can be readily conjugated to exquisitely cell-specific aptamers. The figures below display flow cytometry images of CCRF-CEM and K562 cells treated with sgc8c-7 conjugateand LIB-7 conjugate and MTS results showing the specific cytotoxicity of sgc8c-7 towards target cancer cells by treating CCRF-CEM and K562 cells with LIB-7 (LIB = random library) and sgc8c-7, respectively (in order of appearance).